Injection Medical Device Giredhi Hyaluronic Acid
Tsanangudzo Pfupi:
Iyo yakakwirira-kuchena biomaterial inowanzoshandiswa seviscoelastic supplement iyo inogona kubaiwa mugomba rejoint kuti ibvise kuzvimba kwemajoini, kuderedza marwadzo, uye kunatsiridza kufamba kwemajoini.
Product Characteristics
| Product Name | Sodium Hyaluronate (SH-MDI) |
| INCI | (C14H20NNaO11)n |
| CAS | 9067-32-7 |
| Chitarisiko | Chichena kana Chinenge Chichena, Hupfu kana Granule |
Sodium hyaluronate chimwe chezvinhu zvakakosha zve synovial fluid.Ichi chikamu chine anti-bradykinin uye anti-protease mabasa, izvo zvinogona kunyatso kuvharidzira kufara kwe synovium uye iyo inokonzera marwadzo ekugamuchira uye manzwiro emagetsi kuti abvise marwadzo emajoini.Mushure meiyo exogenous jekiseni yesodium hyaluronate, inogona kuvhara pamusoro pearticular cartilage, kugadzira chinodzivirira, uye kuderedza kukweshera kwemajoini uye kurwadziwa.
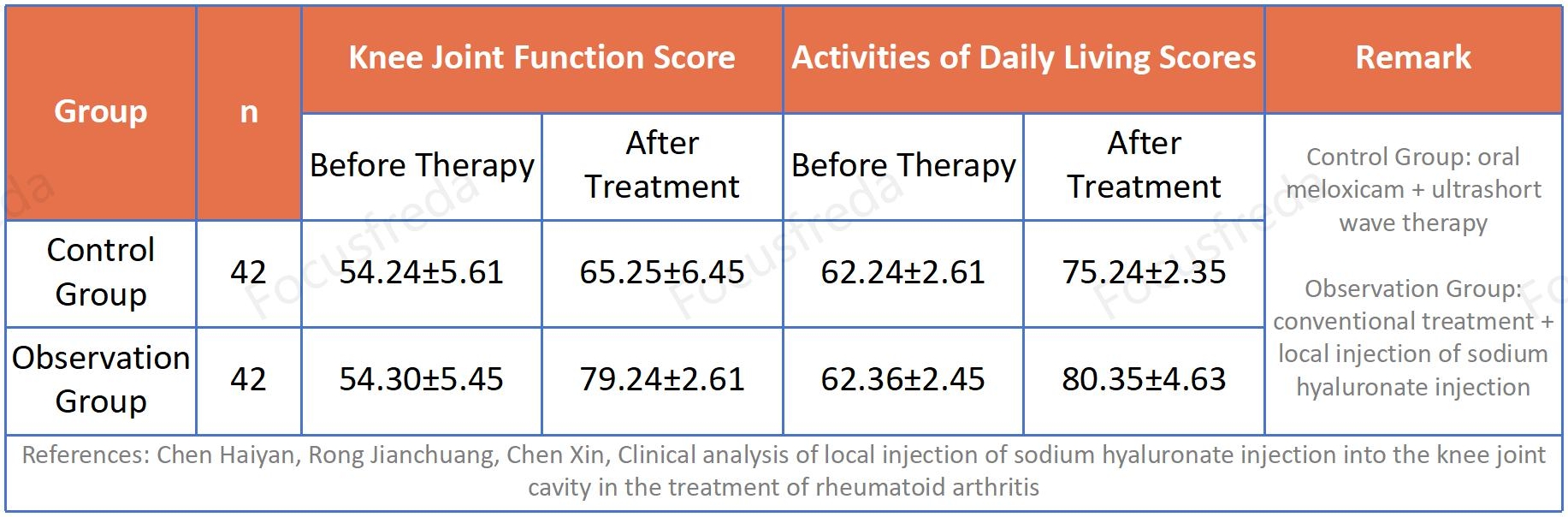
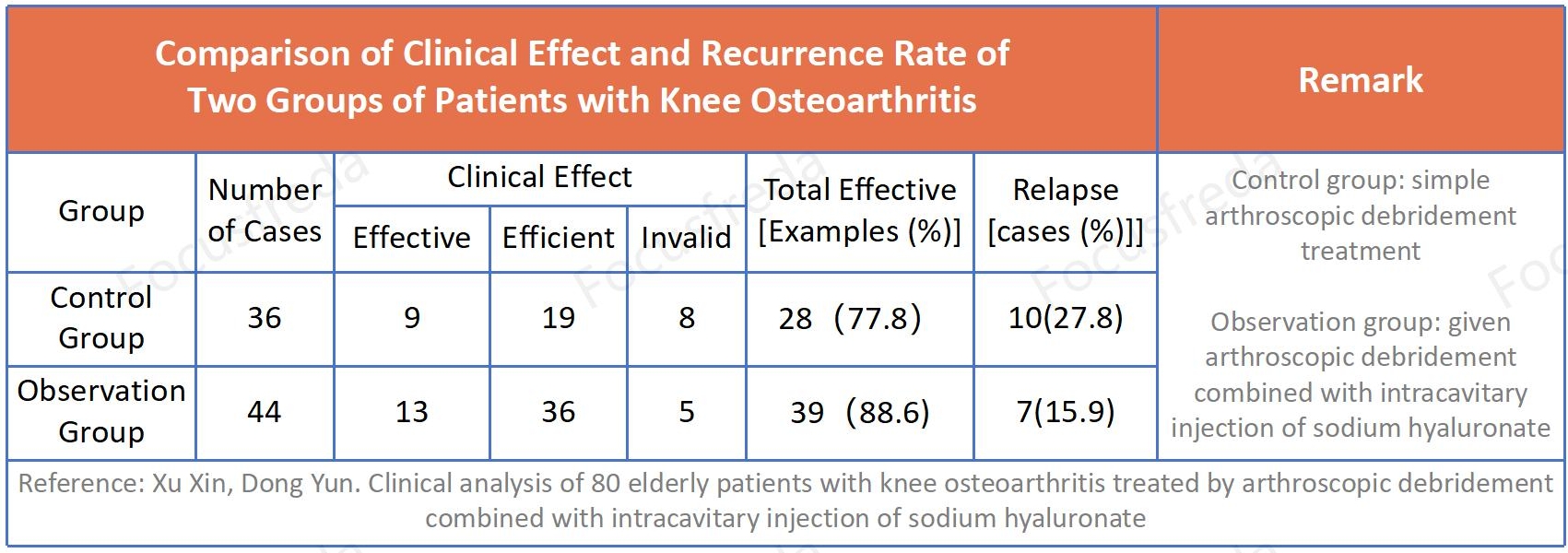
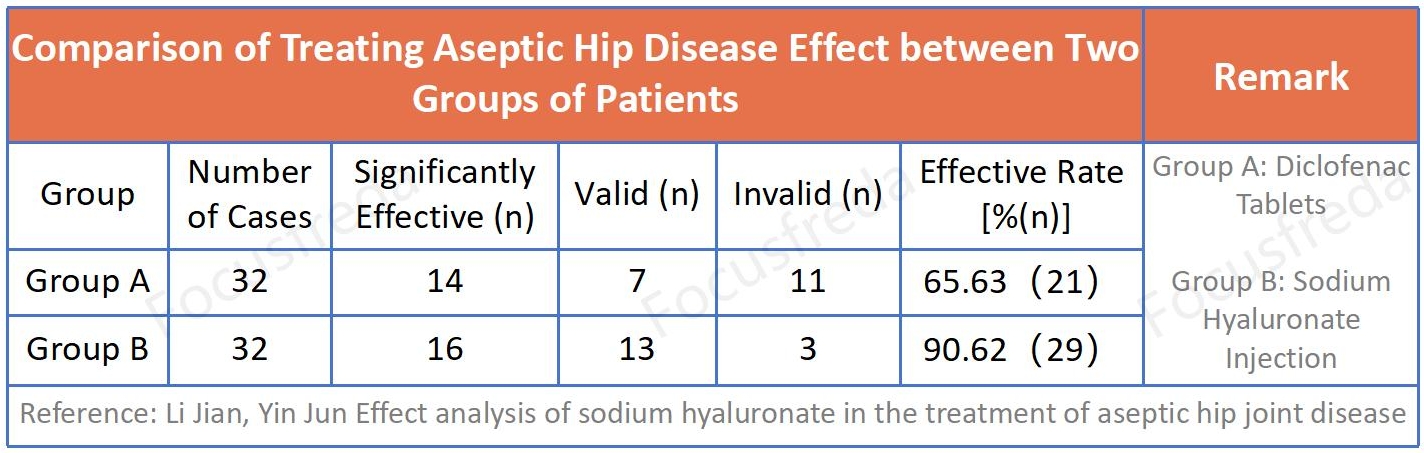
Clinical Research
Chigadzirwa Specification

| Zvichienderana nezvido zvako, isu tichaona zvinotevera zviratidzo kune wese mutengi: | ||
| Identification | Kuonekwa kweSolution | Nucleic Acids |
| PH | Intrinsic Viscosity | Molecular Weight |
| Mapuroteni | Kurasika paKuomesa | Chlorides |
| Iron | Utachiona Hunoverengera | Utachiona Hunoverengera |
| Staphylococcus Aureus | Pseudomonas Aeruginosa | Bacterial Endotoxins |
| Hemolysis | Hemolytic Streptococci | Ethanol Residues |
Application Rage
| Osteoarthritis Kurapa |
| Kupisa uye Kugadziriswa Kwemaronda Kusingaperi |
| Wrinkle uye Fine Line Kuzadza |
| Facial Contour Kuzadza uye Kuumbwa |
| Gadzira Tsono inopenya Mvura |
| Mafuta Grafting Auxiliary |
| Soft Tissue Kugadzirisa uye Regeneration Accelerator |
| Injectable giredhi sodium hyaluronate ine huwandu hwakasiyana hwekushandisa munzvimbo dzekurapa uye zvekuzora, kunyanya kushandisa iyo inonyorovesa, yekuzora uye yekuzadza zvivakwa. |
Storage Conditions
| <1.9m³/kg | Chengetedza munzvimbo yakasimba, isina chiedza, inotonhorera uye ine rima |
| 1.9–3.4m³/kg | Chengetedza munzvimbo yakasimba, isina chiedza, tembiricha iri pasi pe10 ℃ |
| >3.4m³/kg | Chengetedza munzvimbo yakasimba, isina chiedza, tembiricha iri -20~-10℃ |
Package
50g / bhodhoro, 100g / bhodhoro, 200g / bhodhoro, Zvimwe Customization
Sherufu Hupenyu
Kazhinji 2 makore
Ingredients
Hyaluronic Acid & Tremella Fuciformis Polysaccharide
Collagen uye Chondroitin Sulfate
Ectoin uye Sodium Polyglutamate
Taura nesu
 Kero
Kero
 Email
Email

© Copyright - 2010-2023 : All Rights Reserved.Hot Products - Sitemap
Freda Sodium Hyaluronate Powder, Sodium Hyaluronate Powder, Chikafu Giredhi Sodium Hyaluronate, Yakaiswa Sodium Hyaluronate, Chikafu Giredhi Sodium Hyaluronate Powder, Sodium Hyaluronate Chimiro,